Why Knockout Cell Lines Save Weeks in Functional Research

Anyone who has ever built a CRISPR knockout knows the real slowdown in functional studies doesn’t happen during the experiment—it happens before the experiment ever begins.
Most delays come from chasing a model that refuses to behave: inconsistent editing, fragile clones, ambiguous sequencing, and validation cycles that stretch far longer than expected.
And in fast-moving projects—pathway mapping, mechanism exploration, target validation—waiting six to twelve weeks for a model can easily derail timelines.
This article takes a practical, lab-first look at why knockout models save so much time, when they matter most, and how they reduce scientific risk—not just waiting time.
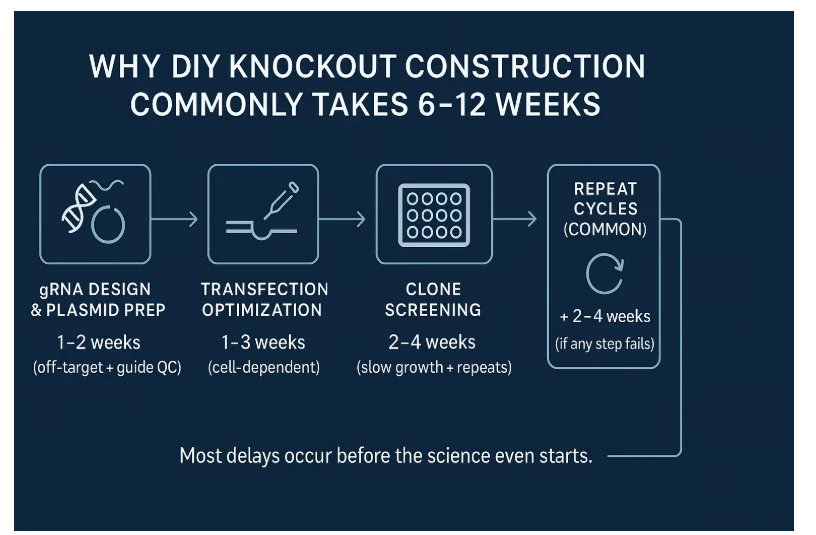
The Hidden Reality: Why DIY KO Construction Takes Longer Than Planned
Researchers often underestimate how fragile a CRISPR workflow can be.
Below is the reality behind each “simple step” that quietly turns a 4–6 week plan into a 10–12 week ordeal.
gRNA Design → Where Delays Begin Unnoticed
Designing a guide takes minutes.
Getting a guide that edits cleanly in your cell line is another story entirely.
A single misplaced PAM or repair hotspot can cause:
- repeated in-frame indels
- mosaic editing
- inefficient allelic disruption
You don’t notice the problem early.
You notice it when you’re already weeks deep into clone screening.
Transfection Challenges and Why Many Labs Turn to KO Cell Lines
Even experienced labs struggle with transfection volatility:
A condition that worked in March suddenly fails in April.
Harder cell lines (HepG2, THP-1, U937) often require repeated optimization cycles—each adding days or weeks to the project.
To avoid losing time to these unpredictable steps, many teams now prefer to work with ready-to-use KO cell lines. Instead of re-optimizing transfection conditions, they simply
click here
to start directly with professionally validated models.
Clone Screening → The Part Everyone Underestimates
Real-world screening often looks like this:
- Round 1: mixed alleles
- Round 2: slow growth or clone death
- Round 3: sequencing contradicts Western
- PI: “Should we start over?”
One failed round costs 2–3 weeks, minimum.
Two failures? You’ve lost a quarter.
Validation → The Slowest “Quick Step”
Validation is where timelines break:
- Sanger looks “KO-ish” but isn’t
- NGS turnaround takes a week
- protein still expressed despite frameshift
- compensatory pathways mask phenotype
Validation failures are the most demoralizing—and the most time-expensive.
Why Downstream Experiments Rely on Ready-to-Use Knockout Cell Lines
Pathway assays, drug-response curves, and synthetic lethality studies all depend on having a stable and well-validated knockout model in place.
Without a confirmed KO, downstream experiments simply cannot begin.
For this reason, many research teams now turn to ready-to-use knockout cell lines instead of repeating long construction cycles. They simply click here to start directly with pre-validated models that are ready for functional assays.
Why Knockout Models Actually Save Time (Beyond the Obvious)
KO models don’t make CRISPR faster.
They remove the steps most likely to waste time.
Day 1 = Data Day
With a validated KO, you start immediately:
- WB
- qPCR
- phenotype assays
- pathway readouts
- rescue experiments
You skip the uncertainty phase and begin generating interpretable data.
Removing High-Failure Steps Removes Entire Delays
The steps most likely to blow up timelines—transfection, clone expansion, genotype/protein validation—simply disappear.
KO Models Reduce Scientific Risk, Not Just Waiting Time
A stable KO means:
- fewer failed repeats
- cleaner dose-response curves
- more interpretable pathway behavior
- fewer “false directions” early in the project
- higher confidence in negative results
Time is important.
But scientific stability is often more important.
Faster Decisions = Faster Research
KO models accelerate:
- preliminary insights
- mechanistic interpretation
- go/no-go decisions
- publication readiness
Avoiding the wrong direction early often saves more time than any optimization.
Where Knockout Models Make the Biggest Difference
Pathway Mapping
Transient KD often destabilizes signaling pathways.
Stable KOs reduce noise and clarify cascade behavior.
Target Validation
Translational studies cannot afford delays.
KO models let you begin dose-response and rescue assays immediately.
Mechanism Studies
Mechanism research hinges on phenotype clarity.
KO models reveal direction earlier and reduce false leads.
Drug Response / Synthetic Lethality
These assays require stable baselines.
KO models avoid batch drift and transient expression noise.
KO Pools for Early Discovery
Pools provide:
- fast readouts
- biological diversity
- reduced clone artifacts
Best used for:
“Is there a phenotype worth pursuing?”
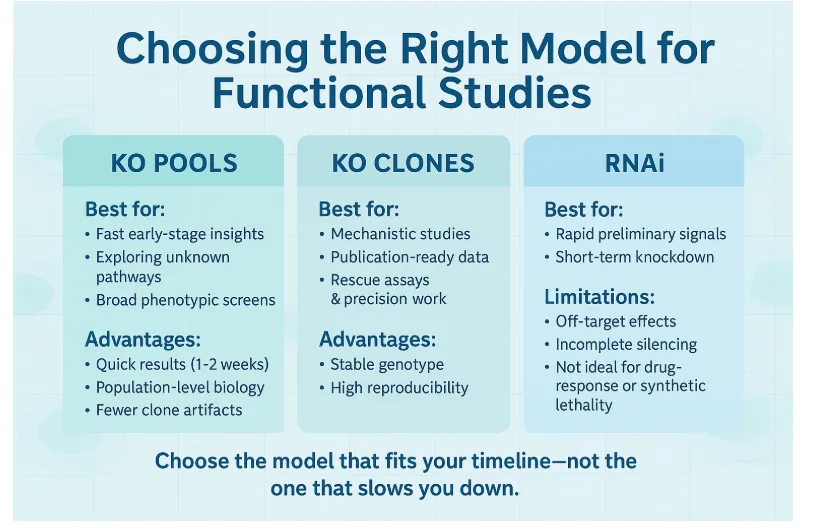
KO Pools vs KO Clones vs RNAi — A Practical Decision Guide
Use KO Pools When:
- You need quick direction
- You’re exploring unknown pathways
- You want first-pass phenotype data
- You need results in ~2 weeks
Use KO Clones When:
- You need publication-level rigor
- You plan rescue assays
- Precision matters
- Reviewer scrutiny is expected
Use RNAi When:
- You only need an initial hint
- Variability is acceptable
- Timeline is <48 hours
But avoid RNAi for:
- synthetic lethality
- drug-response curves
- subtle signaling studies
When a Ready-Made KO Model Is Not the Right Choice
(Added for balance & credibility)
Avoid ready-made KOs when:
- the target gene is essential
- inducible or temporal control is needed
- a specific engineered allele is required
- clone heterogeneity is biologically relevant
This honesty strengthens the article’s trustworthiness.
A Reviewer-Style Checklist: How to Know a KO Model Is Trustworthy
A research-ready KO should have:
- biallelic disruption confirmed by NGS
- protein loss validated via Western
- reproducible phenotype
- at least two independent clones or a pool
- clear QC documentation
- acceptable viability and growth
This is exactly what reviewers look for.
Conclusion: What You Save Isn’t Weeks—It’s Momentum
Most delays in functional studies come not from the assays, but from the model uncertainty phase—editing, screening, validating, and repeating. Knockout models remove the most failure-prone stages and allow experiments to begin immediately. In pathway work, mechanism studies, and early drug discovery, they accelerate not just timelines—but scientific certainty. Research will always demand rigor. But it doesn’t have to move slowly.
